Trelegy Vs Breo
Trelegy Ellipta can increase the risk of developing a yeast infection of the mouth and throat. The approval applies to COPD patients on a fixed dose of Breo Ellipta fluticasone furoatevilanterol who need additional help with airflow obstruction.
 Trelegy Ellipta May Emerge As Major Growth Driver For Glaxosmithkline
Trelegy Ellipta May Emerge As Major Growth Driver For Glaxosmithkline
13 Before starting treatment with Trelegy Ellipta patients should talk to their doctor about all medicines over-the-counter and prescription vitamins and supplements they are taking.

Trelegy vs breo. TRELEGY 10062525 mcg is the only strength approved for COPD. Has anyone switched from Breo Ellipta to Trelegy Ellipta. Trelegy combines the inhaled corticosteroid fluticasone with two long-acting bronchodilators.
Breo Ellipta You may wonder how Trelegy Ellipta compares with other medications that are prescribed for similar uses. I am wondering if you found a difference. One of the events that triggered that alert involved a community pharmacist who mistakenly dispensed Breo Ellipta to a consumer who had been prescribed Incruse Ellipta.
Breo Ellipta should not be used in children and adolescents. TRELEGY delivers rapid and lasting improvement in lung function1. The randomized double-blind active-controlled trial was designed to compare the effects of once-daily single inhaler triple therapy Trelegy Ellipta vs.
How is Trelegy Ellipta to be used. It is not known if Breo Ellipta is safe and effective in children and adolescents younger than 18 years of age. The results also showed that patients who took Trelegy Ellipta could breathe out significantly more air in one second compared to those who took Breo Ellipta which is.
Information was available on 996 of the total population 10355 enrolled in the study. Breo Ellipta is used for COPD and asthma and as follows. Trelegy has less steroid in it.
This risk can be reduced if the patient rinses hisher mouth without swallowing after using the inhaler. Trelegy Ellipta is an orally inhaled medicine that contains three medications in one inhaler. Here we look at how Trelegy Ellipta and Breo.
The approval means the once-a-day single-inhaler therapy will enter the US. TRELEGY is not used to relieve sudden breathing problems and wont replace a rescue inhaler. Breo has been approved for adults 18 years and older while Advair has been approved for both adults and children 4 years and.
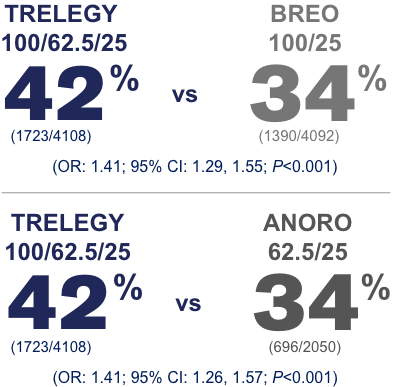
Trelegy Ellipta fluticasone umeclidinium and vilanterol which is used to treat COPD. The new FDA approval was based on data from the IMPACT InforMing the PAthway of COPD Treatment study that demonstrated superiority of Trelegy Ellipta vs RelvarBreo Ellipta and Anoro Ellipta on. Trelegy Ellipta vs.
Both Breo and Advair are prescription drugs that the FDA has approved for long-term management of chronic obstructive pulmonary disease COPD and asthmaThey cannot be used to treat acute flare-ups of asthma or COPD. There were significantly fewer deaths in those receiving triple therapy combination 236 Trelegy compared with the combination of two bronchodilators 319 Anoro but not with the combination of a bronchodilator and an inhaled corticosteroid 264 Breo. Breo Ellipta fluticasone furoatevilanterol and Incruse Ellipta umeclidinium.
Dual therapy Breo Ellipta. Breo is FDA-approved for the treatment of asthma and is a first-line recommended treatment for COPD. TRELEGY is not a rescue inhaler and should not be used for the relief of acute symptoms.
It also applies to patients on Incruse Ellipta umeclidinium and. Limitations of Use TRELEGY is not used to relieve sudden breathing problems and will not replace a rescue inhaler. As early as 15 minutes1.
Vilanterol a long-acting beta-2 agonist LABA and umeclidinium a. Breo Ellipta 10025 is a prescription medicine used to treat COPD. It is a combination of the following 2 inhalers.
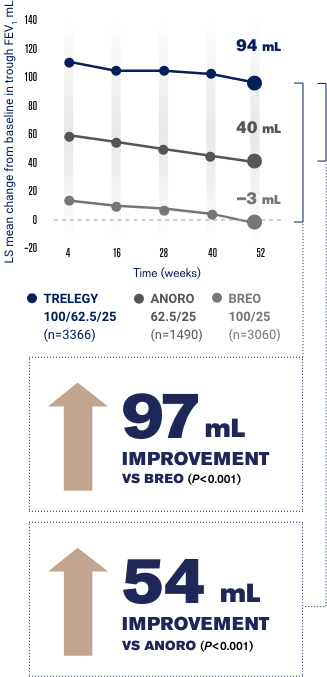
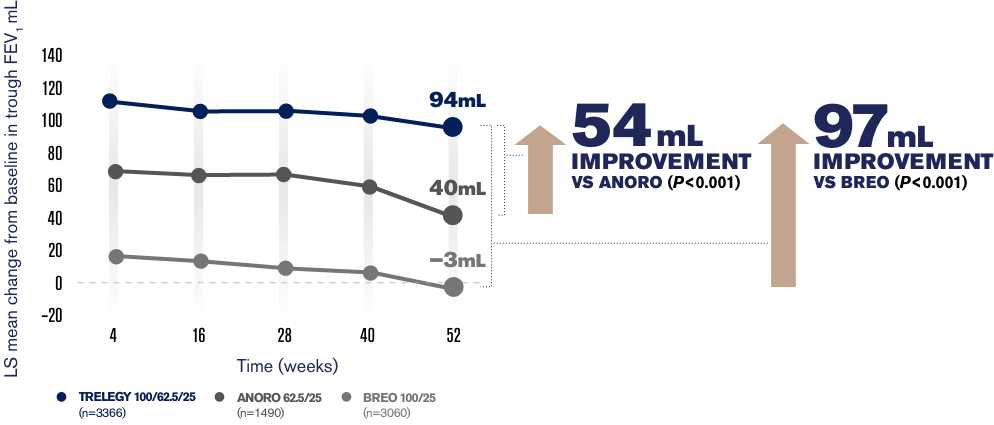
Trelegy reduced flare-ups by 15 compared to Breo over one-year a significant effect. In addition there was a statistically significant 92mL improvement in trough FEV 1 with the Trelegy Ellipta 20062525mcg dose vs Breo Ellipta 20025mcg P. In early 2017 we first alerted healthcare providers to these mix-ups.
COPD is a chronic lung disease that includes. TRELEGY provided improvement in FEV 1 vs BREO as measured by LS mean change from baseline in FEV 1 beginning at 15 minutes on Day 1. TRELEGY should not be used in children younger than 18 years of age.
What Is The Difference. Trelegy Ellipta is a triple combination inhaler containing 100 mcg of fluticasone furoate 625 mcg of umeclidinium and 25 mcg of vilanterol. The recommended dose of Trelegy Ellipta is one inhalation by mouth once daily.
Results of the study showed a statistically significant 110mL improvement in trough FEV 1 with Trelegy Ellipta 10062525mcg compared with Breo Ellipta 10025mcg P. Trelegy has a major difference with the previous two in that it contains 3 active ingredients. The data also showed that Trelegy and Breo the two medications containing the corticosteroid fluticasone furoate lowered the risk of on-treatment mortality compared to Anoro.
The first two are the exact same as Breo fluticasone furoate and vilanterol. Specifically Trelegy decreased this risk by 421 compared to Anoro. BREO 10025 n407 TRELEGY 10062525 n406 24 m L 134 mL PRIMARY ENDPOINT.
Market under the brand name Trelegy Ellipta. COPD360social posts are monitored by Vice President of Patient Experience and COPD360social Community Manager Bill Clark as well as staff Respiratory Therapists. The third is umeclidinium which is in a class of drugs used for COPD but not asthma called.
How do Breo and Advair work. Trelegy on the other hand is only FDA approved for the treatment of COPD but only as a second-line option according to the Global Initiative for Chronic Obstructive Lung Disease GOLD guidelines. However off-treatment data should be analyzed to fully understand the benefits in mortality risk.
CHANGE FROM BASELINE IN TROUGH FEV 1 AT W EEK 2 4 110 mL IMPROVEMENT VS BREO P 0001 90 120 150 180 60 30 0 90 120 150 180 60 0 L S m e a n c h a n g e fr o m b a s e l i n e m L BREO 20025 n 406 TRELEGY 200625 25 n 408 76mL 168mL 92 mL IMPROVEMENT.
.png?auto=format) Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
.png?auto=format) Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
 Trelegy Ellipta Improves Lung Function In Patients With Uncontrolled Asthma Mpr
Trelegy Ellipta Improves Lung Function In Patients With Uncontrolled Asthma Mpr
 Fda Oks Glaxo S Inhaler First One To Combine 3 Medicines The Spokesman Review
Fda Oks Glaxo S Inhaler First One To Combine 3 Medicines The Spokesman Review
 Advair Vs Breo Vs Trelegy What Is The Difference
Advair Vs Breo Vs Trelegy What Is The Difference
 Advair Vs Breo Vs Trelegy What Is The Difference
Advair Vs Breo Vs Trelegy What Is The Difference
 Breo Vs Trelegy What Is The Difference
Breo Vs Trelegy What Is The Difference
 Innoviva Gsk Project Trelegy Ellipta Meets Main Endpoint In Asthma Pivotal Trial Drug Delivery Business
Innoviva Gsk Project Trelegy Ellipta Meets Main Endpoint In Asthma Pivotal Trial Drug Delivery Business
 Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
 Advair Vs Breo Vs Trelegy What Is The Difference
Advair Vs Breo Vs Trelegy What Is The Difference
 Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
 Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
Impact And Trials 1 2 Data Trelegy Ellipta Fluticasone Furoate Umeclidinium Vilanterol
Trelegy Ellipta Medicare Coverage And Co Pay Details Goodrx

Comments
Post a Comment