Novartis Ms Drug
The Novartis multiple sclerosis portfolio includes Gilenya fingolimod an S1P modulator which is indicated in European Union for the treatment of adult patients and children and adolescents 10 years of age and older with RMS. Mayzent is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors.
 Novartis Takes Aim At Roche S Star Ms Drug Euronews
Novartis Takes Aim At Roche S Star Ms Drug Euronews
Novartis Wins Patent Battle for its Blockbuster MS Drug Gilenya Published.
Novartis ms drug. Food and Drug Administration FDA approval to repurpose an 11-year-old blood cancer drug against multiple sclerosis as the Swiss drugmaker takes on. Swiss pharma giant Novartis AG NVS announced that the European Commission EC has approved its multiple sclerosis drug Mayzent siponimod. Aug 18 2020 By Alex Keown Novartis is breathing a sigh of relief after a US.
Roche has hit back at Novartis newly-approved multiple sclerosis drug Kesimpta with new data from its already-marketed blockbuster Ocrevus. Novartis Wins EU Thumbs Up for MS Treatment Kesimpta could become the first and only self-administered targeted B-cell therapy for patients in Europe with relapsing forms of multiple sclerosis. Today Gilenya is widely recognized by doctors with over 283000 patients treated with Gilenya to date.
September 11 2020. Entresto was approved in 2017. Federal judge upheld Novartis dosage regiment patents for its multiple sclerosis drug Gilenya.
Approved in 2010 for RRMS Gilenya earned Novartis nearly 32 billion last year even in the face of stiff competition from rival drugs made by Biogen Inc Sanofi SA and Teva Pharmaceutical Industries Ltd. Novartis multiple sclerosis drug has been given the green light by the European Medicines Agencys CHMP scientific committee paving the way for a likely approval in the coming weeks. Novartis AG undercut the price on its established multiple sclerosis treatment with a pill to be introduced next week a sign that change may be afoot in one of the areas of medicine most plagued.
Last week Novartis was touting positive data showing its repurposed leukemia drug ofatumumab outpaced Sanofis Aubagio in a multiple sclerosis. Basel August 20 2020 Novartis today announced that the US Food and Drug Administration FDA has approved Kesimpta ofatumumab formerly OMB157 as an injection for subcutaneous use for the treatment of relapsing forms of multiple sclerosis RMS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in. In the United States Gilenya is approved for the treatment of adults with relapsing forms of MS to include clinically isolated syndrome CIS relapsing remitting disease.
Studies showed ofatumumab could significantly reduce disease relapses compared with Sanofis Aubagio medicine. EU Approval Verdicts Near For A Bumper Crop Of New Drugs. Gilenya is the 3rd most prescribed MS disease modifying treatment worldwide.
Novartis gains EU approval for relapsing MS drug Kesimpta The drug reduces the number of confirmed relapses in MS patients The European Commission EC has approved Novartis Kesimpta for the treatment of relapsing forms of multiple sclerosis RMS in adults with active disease. Ofatumumab is a critical drug in Novartiss pipeline expected to challenge Roches Ocrevus a top multiple sclerosis medicine. The drug is approved for the treatment of adult patients with secondary progressive multiple sclerosis SPMS with active disease.
Mayzent was approved for adults with relapsing forms of the disease including clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease SPMS the FDA said. Novartis has priced the treatment at 88000 annually head of pharmaceuticals Paul Hudson told Reuters a level exceeding some other MS drugs and one that may draw scrutiny from payers. Novartis is committed to bringing innovation to China and to reimagining care for patients.
ZURICH Reuters - Novartis on Thursday won US. Genmab licensed the drug to GlaxoSmithKline and Novartis went on to gain rights to the drug as part of a complex asset swap deal between the two companies in. Basel March 27 2019 - Novartis today announced that the US Food and Drug Administration FDA has approved Mayzent siponimod for the treatment of adults with relapsing forms of multiple sclerosis including secondary progressive multiple sclerosis SPMS with active disease relapsing remitting multiple sclerosis RRMS and clinically isolated syndrome CIS.
For Novartis siponimod would bolster a franchise led by the pharmas aging top-seller Gilenya fingolimod. Unlike Ocrevus which requires an infusion and monitoring ofatumumab is a self-administered injection.
 Novartis Showcases New Data On Ms Drugs Mayzent And Kesimpta At Actrim
Novartis Showcases New Data On Ms Drugs Mayzent And Kesimpta At Actrim
 Nice Changes Its Mind On Novartis Progressive Ms Drug Mayzent
Nice Changes Its Mind On Novartis Progressive Ms Drug Mayzent
 Novartis Makes New Ms Drug Cheaper Than Its Older Option Heat Health Evaluations Applied Therapeutics
Novartis Makes New Ms Drug Cheaper Than Its Older Option Heat Health Evaluations Applied Therapeutics
Regulators Expand Review Time For Novartis Ms Drug Pharmatimes
 Fda Approves Generics Of Novartis Multiple Sclerosis Drug Gilenya
Fda Approves Generics Of Novartis Multiple Sclerosis Drug Gilenya
 Novartis Gilenya Fails Phase Iii Trials As A Drug For Multiple Sclerosis
Novartis Gilenya Fails Phase Iii Trials As A Drug For Multiple Sclerosis
Novartis Ms Drug Improves Outcomes Pharmafile
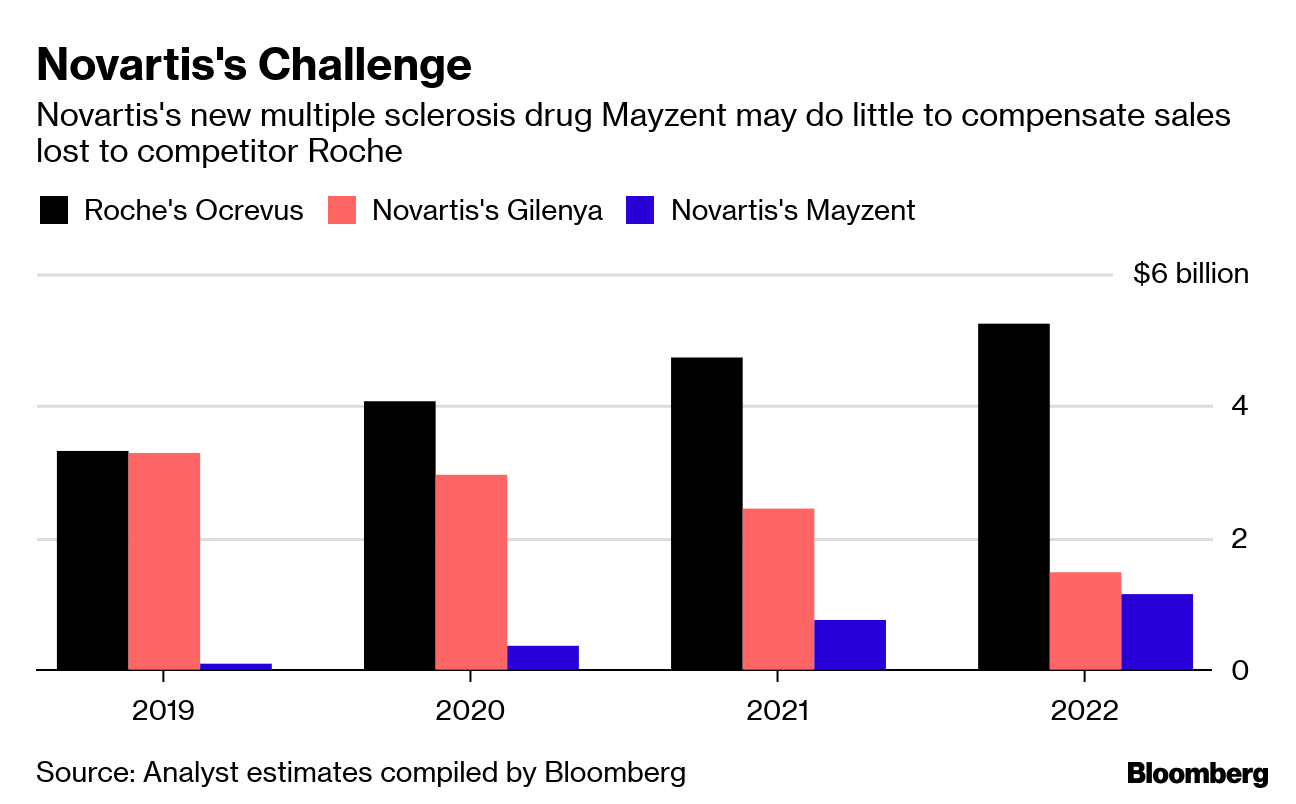 Novartis Makes New Ms Drug Cheaper Than Its Older Option
Novartis Makes New Ms Drug Cheaper Than Its Older Option
 China Approves Older Novartis Ms Drug Caixin Global
China Approves Older Novartis Ms Drug Caixin Global
Fda Updates Gilenya Label After Safety Review Pmlive
 Ectrims Novartis Reinvented Leukemia Drug Bests Sanofi S Aubagio In Slashing Ms Relapses Fiercepharma
Ectrims Novartis Reinvented Leukemia Drug Bests Sanofi S Aubagio In Slashing Ms Relapses Fiercepharma
 Novartis Says Ms Drug Cut Risk Of Disability Advance In Study Fortune
Novartis Says Ms Drug Cut Risk Of Disability Advance In Study Fortune
 Cost Effectiveness Watchdogs Skewer Novartis Ms Drug Mayzent And J J S Depression Spray Spravato Fiercepharma
Cost Effectiveness Watchdogs Skewer Novartis Ms Drug Mayzent And J J S Depression Spray Spravato Fiercepharma
 Siemens Novartis Team Up On Ms Drug Development Drug Discovery And Development
Siemens Novartis Team Up On Ms Drug Development Drug Discovery And Development
Comments
Post a Comment